Open Disclosure Policy
Purpose
Open disclosure is a patient and consumer right and an essential professional requirement of healthcare delivery in Australia. It is directed and guided by The Australian Open Disclosure Framework.
Open Disclosure will be managed at Templestowe Day Surgery (TDS) through thorough clinical review and investigation of adverse events and related outcomes, with a focus on clinical risk, quality improvement and prevention of recurrence.
This policy outlines all required practices to be implemented in this instance at TDS. All TDS staff will be encouraged and supported to use the CAR system to report clinical incidents, near misses and adverse events.
Scope
This policy applies to all staff at TDS, including all credentialed medical and other practitioners.
Definitions
Adverse event: an incident that results, or could have resulted, in harm to a patient or consumer. A near miss is a type of adverse event.
Australian Open Disclosure Framework: provides a framework for health service organisations and clinicians to communicate openly with patients when health care does not go to plan.
Near miss: an incident or potential incident that was averted and did not cause harm, but had the potential to do so.
Open disclosure: an open discussion with a patient and family/carer about an incident that resulted in harm to the patient while receiving health care. The criteria of the open disclosure process include an expression of regret, and a factual explanation of what happened, the potential consequences, and the steps taken to manage the event and prevent recurrence.
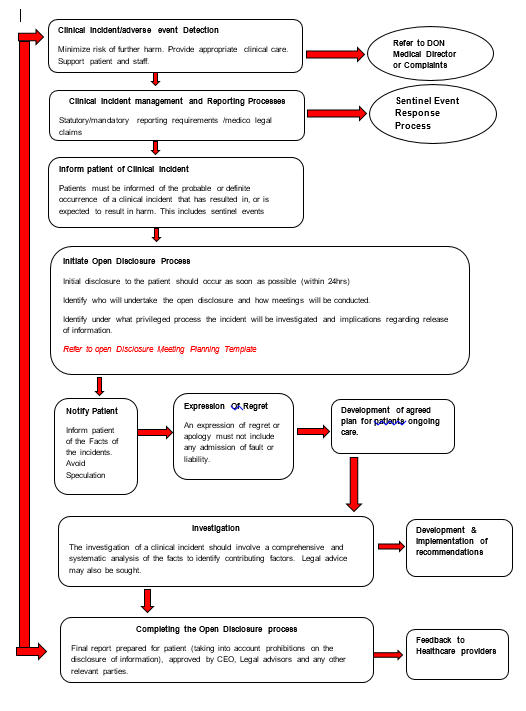
KEY ELEMENTS OF THE OPEN DISCLOSURE PROCESS (see Flowchart in Appendix A)
Patients and carers will be provided with information about what incidents and concerns are, and how to report them.
Prompt clinical care of the patient will be provided following an adverse event to prevent further harm
Staff will be supported at all times by their colleagues and TDS managers both personally (emotionally) and professionally, including through appropriate training, preparation and debrief.
Patient and clinician privacy and confidentiality must be maintained at all times.
Staff may report adverse events anonymously.
Adverse events will be raised with the senior Dr as soon as practicable following occurrence.
Adverse events will be documented using the Corrective Action Report (CAR) system on the TDS Google Drive.
The senior clinician (Dr or DON/senior Registered Nurse) on duty will assess the incident for severity of harm and level of response and document in the CAR.
The relevant professional indemnity insurers will be informed if there is a perceived risk of material, financial or reputational damage due to the incident.
Upon notification of an adverse event the senior clinician will:
- provide assistance for the reporting staff member
- acknowledge the event with the patient and family and provide an appropriate expression of regret or apology. This should include the words ‘I am sorry’ or ‘we are sorry’, but must not contain speculative statements, admission of liability or apportioning of blame. See Saying Sorry: a guide to apologising and expressing regret in open disclosure for further ideas (www.safetyandquality.gov.au/opendisclosure)
- assist staff member to commence an investigation
- determine the need for reporting to further authorities
- notify relevant staff of the incident and their need for involvement in investigation and provide ongoing support
- ensure privacy and confidentiality of patients and clinicians are observed at all times.
The patient and family must at all times be:
- Treated with empathy, respect and consideration
- Supported in a manner appropriate to their needs.
- Preparing for open disclosure
- Hold a team discussion to prepare for open disclosure
- Consider who will participate in open disclosure
- Appoint an individual to lead the open disclosure based on previous discussion with the patient, their family and carers
- Gather all the necessary information
- Identify the health service contact for the patient, their family and carers (if this is not done already)
- Negotiate with the patient, their family and carers or nominated contact person
- the formality of open disclosure required
- the time and place for open disclosure
- who should be there during open disclosure
- Provide written confirmation
- Provide a health service contact for the patient, their family and carers
- Avoid speculation and blame
- Engaging in open disclosure
- Provide the patient, their family and carers with the names and roles of all attendees, verbally and in writing
- Provide a sincere and unprompted apology or expression of regret including the words I am or we are sorry
- Clearly explain the incident, including a factual explanation about what happened in an open and honest manner. Ensure communication is appropriate to the participant’s situation, culture and level of understanding. Avoid speculation.
- Give the patient, family and carers the opportunity to tell their story, exchange views and observations about the incident and ask questions
- Encourage the patient, family and carers to describe the personal effects of the adverse event. It is important for the patient, family and carers to know their views and concerns are listened to, understood and considered.
- Agree on, record and sign an open disclosure plan
- Assure the patient, family and carers that they will be informed of further investigation findings and recommendations for system improvement
- Offer practical and emotional support to the patient, their family and carers
- Support staff members throughout the process
- Maintain good verbal and written communication throughout the open disclosure process
- Providing follow-up
- Ensure follow-up by senior clinicians where appropriate
- Agree on future care
- Share the findings of investigations and the resulting practice changes
- Offer the patient, family and carers the opportunity to discuss the process with another clinician (e.g. a general practitioner)
- ongoing support including reimbursement of out-of-pocket expenses incurred as a result of the adverse event
- assurance that any necessary follow-up care or investigation will be provided promptly and efficiently
- clarity on who will provide ongoing care resulting from the adverse event
- contact details for any relevant service they wish to access information about how to take the matter further, including any complaint processes available to them including the Victorian Health Complaints Commissioner if necessary (www.hcc.vic.gov.au/public/about-complaints or phone 1300 582 113).
- Completing the process
- Reach an agreement between the patient, their family and carers and the clinician, or provide an alternative course of action
- Provide the patient, their family and carers with final written and verbal communication, including
- how feedback will be provided on the investigation findings, by whom and in what timeframe.
- any changes made to minimise the risk of recurrence.
- Communicate the details of the adverse event, and outcomes of the open disclosure process, to other relevant clinicians
- Complete the evaluation surveys
- The event and outcome must be published on the TDS website in accordance with the Health Services (Private Hospitals and Day Procedure Centres) Regulations 2013
- Maintaining documentation
- Provide a copy provided to the patient, family and carers.
- Maintain a separate record of the open disclosure process
Staff must have training at commencement, with annual updates, in management of incidents, adverse events and the open disclosure process
All education will be documented by the DON and records maintained.
All staff must be prepared to participate in open disclosure.
Reporting and AUDIT
The complaints and Informed consent processes will be linked to the Open Disclosure Framework to ensure related incidents are collected, managed and reviewed for ongoing quality improvement and risk management.
Incidents, including open disclosure events will be tabulated and compiled on an ongoing basis by the DON.
All serious incidents and Open Disclosure events will be reported quarterly at MAC meetings to ensure appropriate follow up has occurred.
The incident management and open disclosure systems will be audited at least annually to ensure they are consistent with the Australian Open Disclosure Framework and are being implemented appropriately at TDS.
Incident data will be analysed, trends and opportunities for improvement identified.
References
Australian Commission on Safety and Quality in Health Care. Open disclosure standard. Sydney: ACSQHC; 2008.
NSQHSS Day Procedure Services Accreditation Workbook (October 2017)
Supporting Documents
Minutes of Medical Advisory Committee meetings
Minutes of Board of Management Meetings
Open Disclosure Framework http://www.safetyandquality.gov.au/wp-content/uploads/2013/03/Australian-Open-Disclosure-Framework-Feb-2014.pdf
Open Disclosure Training http://vhimsedu.health.vic.gov.au/opendisclosure/welcome.php
Open Disclosure Consumer Brochure
Health Services (Private Hospitals and Day Procedure Centres) Regulations 2013
TDS CAR Form
TDS CAR Register
Appendix A: The Open Disclosure Process